- within Intellectual Property topic(s)
- with readers working within the Pharmaceuticals & BioTech industries
A Supplementary Protection Certificate (SPC) is an intellectual property right available for active ingredients of human and veterinary medicinal products requiring marketing authorisation1.
SPCs were introduced as a mechanism to compensate patent holders for loss in effective patent term resulting from the time taken to receive marketing authorisation for such products2. However, regulatory delay is not of itself sufficient to justify the grant of SPCs. Some of the complexities in the system are explored further below.
The relevant regulation provides that an SPC can be granted only for an "active ingredient". This has been held to exclude substances that may enable or enhance the activity of a therapeutic ingredient, but which have no therapeutic effect of their own on the human or animal body. Despite the clinical testing (and consequent regulatory delay) involved in developing such auxiliary substances, the CJEU3 held that they do not qualify for an SPC. Similarly, it is not currently possible to obtain SPC protection for a medical device, irrespective of whether or not the marketing of such a device has been subject to regulatory delay.
The highest tribunal hearing disputes involving SPCs for EU member states is the Court of Justice of the European Union (CJEU). Historically there have been numerous referrals to the CJEU on points of law relating to SPCs. These decisions are influential also in non-member states including the UK and Switzerland. Some of the key decisions are discussed below.
Where are SPCs available?
SPCs are national rights: at present there is no such thing as a Europe-wide SPC. Accordingly, individual applications must be made to national patent offices in countries where SPC protection is desired, although a national SPC application may be based on a European Unitary Patent in those countries in which the Unitary Patent takes effect. Additional information on the Unitary Patent is provided on our website.
SPC protection is available in all EU member states, namely:
Austria, Belgium, Bulgaria, Croatia, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, Netherlands, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, and Sweden.
SPCs in these countries are governed by EC Regulation 469/2009 ("the SPC Regulation" – excerpts of which are included as Annex 1).
SPC protection is also available in the following non-EU States:
- United Kingdom: SPCs in the UK are available on effectively the same terms as the EU SPC Regulation, which was transposed into national law at the end of the Brexit implementation period (31 December 2020)4. See separate section below: Additional considerations in the UK post-Brexit.
- Norway and Iceland: These countries are members of the European Economic Area (EEA), but not of the EU. However, the governing legislation remains the EU SPC Regulation.
- Switzerland: Swiss SPCs are governed by national legal provisions which are based on the EU SPC Regulation. An SPC issued in Switzerland will also automatically take effect in Liechtenstein5.
- Albania, Bosnia & Herzegovina, North Macedonia, and Serbia: These are non-EU/EEA countries which may nonetheless be covered by a European patent application granted by the EPO. SPC protection is available in these countries under national legal provisions.
Similar provisions also exist in neighbouring jurisdictions including Russia and Ukraine, and in other countries worldwide. Please ask your J A Kemp contact separately regarding rights in these jurisdictions.
What scope of protection is provided by the SPC?
The scope of an SPC is limited to the product of the relevant marketing authorisation. It protects that product to the same extent as the patent on which the SPC is based ("the basic patent"). For example, if the basic patent only covers a method of manufacturing or using the product, then the SPC will be similarly restricted.
Conversely, if the basic patent covers the product per se, the SPC will cover any use of the product which is approved for therapeutic use before the SPC expires. Subsequent marketing authorisations made after grant of an SPC will therefore extend the scope of the SPC, even when the later marketing authorisation is obtained by an entity unconnected with the owner of the SPC. Also, subject to the scope of the basic patent, an SPC will cover all subsequently authorised combinations of active ingredients containing the product6.
The product of the marketing authorisation has long been established to encompass therapeutically equivalent salts and esters of small molecule drugs7 provided of course that they are covered by the basic patent. The situation is less clear for active ingredients which are biological molecules. A decision of the Norwegian Court of Appeal8 following an advisory opinion of the EFTA court9 recognised that it would be desirable for therapeutically equivalent variants of a biologic product to be covered by an SPC, but provided little guidance as to the extent of such coverage. It is to be expected that this question will be referred to the CJEU.
Are there any exceptions from infringement?
The EU/EEA has introduced a so-called "SPC manufacturing waiver" which came into effect on 1 July 2019. The waiver allows manufacture of medicines protected by SPCs for the exclusive purpose of export to markets outside the EEA. Stockpiling for post-expiry use in the EU/EEA is permitted within the final 6 months of the SPC term. It applies to all SPCs applied for after 1 July 2019. Transitional provisions may be relevant for earlier SPCs10. An equivalent waiver has been transposed into UK law and applies to manufacture for export to markets outside the UK and the EEA. More information on the EU/EEA and UK waivers is provided in our separate briefing on the SPC manufacturing waiver.
No waiver presently exists in Switzerland.
What additional term is provided by the SPC?
An SPC takes effect at the end of the normal expiry term of the basic patent on which it is based, provided that the patent is maintained up to that point.
For EU/EEA member states, the SPC will expire at whichever is the earlier of:
- 15 years from the first Marketing Authorisation in the EU/EEA11
- 5 years from the expiry of the basic patent
The effective maximum term is therefore 5 years in addition to the term of the basic patent.
For non-EU/EEA member states, the term is usually determined by reference to the local marketing authorisation. For example, the term of a Swiss SPC is determined by reference to the date of the Swiss marketing authorisation. Since this can issue later than the EU/EEA authorisation, the Swiss SPC for a medicinal product may have a longer term than the corresponding SPCs in the EU/EEA countries. However, as currently transposed from the EU Regulation, the law in the UK is such that term will be calculated based on the first authorisation for the product in either the EU/EEA or the UK.
It is possible to extend the term of an SPC in the EU/EEA, the UK and Switzerland by a further 6 months by providing clinical results obtained from an agreed paediatric investigation plan (PIP). The PIP must be agreed for the relevant territory / country. The request for extension may be filed at any time up to 2 years before normal SPC expiry. The UK follows essentially the same legislation, which has been transposed into UK law. Further information is provided in our separate briefing on Paediatric extensions to Supplementary Protection Certificates in the EU / EEA and UK.
Switzerland has similar legislation but imposes some additional requirements. Please ask your usual J A Kemp contact for more information on paediatric extensions in Switzerland.
Who should apply for the SPC?
The applicant for the SPC must own the basic patent, but need not hold the relevant marketing authorisation. Thus, it is possible to secure an SPC based on a marketing authorisation held by a third party12.
When should the SPC application be filed?
An application for an SPC must be filed with the national patent office of the country concerned within the later of:
- 6 months from the date on which the first authorisation to place the product on the market is granted in that country; or
- 6 months from the date of grant of the basic patent.
If the basic patent expires before marketing authorisation is achieved, it is not possible to secure an SPC13.
What are the substantive requirements for obtaining SPCs?
The requirements for grant of an SPC are set out in Article 3 of the SPC Regulation.
- Article 3(a) requires that the product be "protected" by a basic patent.
- Articles 3(b) and 3(d) require that the SPC be based on the first valid authorisation to place the product on the market as a medicinal product.
- Article 3(c) requires that the product has not already been the subject of an SPC.
Although these requirements may appear relatively simple, each has been subject to multiple referrals to the CJEU. More detailed discussion is provided below.
What is meant by "protected" by a basic patent?
Perhaps counter-intuitively, it is not sufficient for the purposes of Article 3(a) that the product is embraced by the claims of the basic patent. Denied such a comparatively simple "infringment test", the CJEU has been asked repeatedly to clarify the Article 3(a) requirement, resulting in a long line of decisions starting with "Medeva"14 and "Eli Lilly"15 though "Teva"16 and "Royalty Pharma"17, and most recently "Teva/Merck"18.
The consensus view is that the CJEU has arrived at a relatively strict interpretation of Article 3(a), with essentially a two-part test to determine compliance:
- The product must, from the point of view of a person skilled in the art and in the light of the description and drawings of the basic patent, necessarily fall under the invention covered by the basic patent.
- The person skilled in the art must be able to identify the product specifically in the light of all the information disclosed by that patent, on the basis of the prior art at the filing date or priority date of the patent concerned.
In this context, the interpretation of Article 3(a) is perhaps best viewed as a spectrum, as illustrated below:
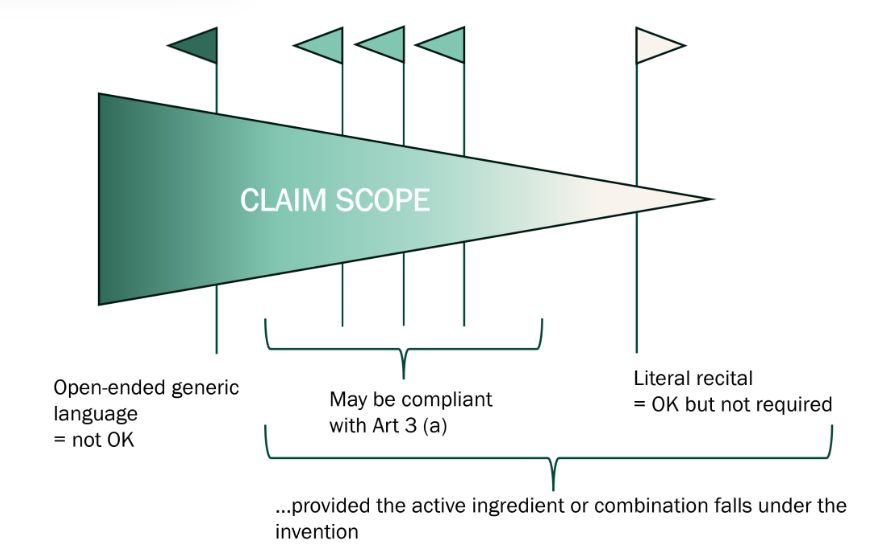
Best practice: select a patent in which the active ingredient or combination of active ingredients is as close as possible to the central inventive concept relied upon for patentability, and which includes at least a dependent claim focused as narrowly as possible on the active ingredient or combination of active ingredients.
Although they have been held to apply to SPCs for single active ingredients, many of the key CJEU decisions explored whether or not Article 3(a) is satisfied in the particular context of products which comprise combinations of active ingredients. This leads to some additional subtleties for such products.
Specifically, a patent which claims product A and does not mention combination therapies cannot support an SPC for combinations of active ingredients containing A, e.g. an SPC for A+B, even though the claims of the patent would embrace combinations of A with any other ingredient. Further, even if a patent claiming A does mention a combination therapy A+B, the combination A+B must constitute an "invention" that goes beyond the invention of monotherapy A alone. This requirement is likely to be satisfied if there is a synergistic interaction between the two ingredients, although it may be possible to argue that there is an "invention" in the combination also under other circumstances 18.
The CJEU1 has also confirmed that a patent which claims only a combination of A+B cannot be the basic patent for an SPC for A alone, despite the fact that sale of A may well, under some circumstances, infringe the patent under "contributory infringement" provisions. This remains true even where the marketing authorisation is for a medicine comprising A and includes an indication that A may or should be used together with B.
However, a granted SPC for A will cover the authorised use of a combination A+B, provided the patent claims do not exclude the combination. See also the following section.
What is meant by "a valid authorisation to place the product on the market as a medicinal product"?
The CJEU has confirmed that an SPC for a given product should be based on the first authorisation for a drug containing the product even if this is a combination therapy which includes the product. Thus, for example, an SPC for product A can be based on a patent claiming A and a marketing authorisation for a medicinal product containing A+B2. This may be important for vaccine products, where marketing authorisations often relate to combinations of multiple active ingredients. An SPC granted under such circumstances will cover all products containing product A approved before the SPC expires.
As mentioned above, the marketing authorisation must be for an "active ingredient". If the marketing authorisation expressly designates a substance as having activity against the authorised indication, such a substance will qualify as an "active ingredient" under the SPC Regulation. However, there is some dispute as to whether SPC protection may also be obtained for a substance forming part of an authorised product which has a pharmacological effect in respect of the authorised indication but which is not expressly designated in the marketing authorisation documents as an active ingredient (and rather is formally designated as an "expicient" in those documents). This is the subject of a pending referral to the CJEU3.
Which marketing authorisation is the first Marketing Authorisation?
Article 3(d) of the SPC Regulation requires that an SPC be based on the first authorisation to place a drug on the market as a medicinal product (the earliest marketing authorisation). The proper identification of the earliest marketing authorisation may be an issue when a patent protecting a second or subsequent medical use of a particular drug is used as the basis for an SPC application.
In the Yissum decision4 the CJEU confirmed that a patent to a new medical use of a drug could form the basis of an SPC, but held that the SPC must be based on the earliest marketing authorisation for that drug, even if the earliest authorisation was for a different disease or condition from that specified in the patent. In practice, reference to the earliest marketing authorisation often meant that any resultant SPC would have a zero term, because of the maximum SPC term of 15 years from first marketing authorisation in the EU.
Many were surprised when the landmark "Neurim" decision5 introduced a more generous interpretation taking into account the scope of the claims of the basic patent. Neurim established that the first marketing authorisation under Article 3(d) was the first such authorisation falling within the limits of the protection conferred by the basic patent. This opened the door to SPCs for new uses of known drugs based on downstream patents and later marketing authorisations, provided the patent claims excluded the product as specified by earlier marketing authorisations. There was much debate over how broadly the principles outlined in Neurim should be applied, with the various national patent offices diverging to a significant extent6.
Perhaps inevitably, this led to additional referrals in "Abraxis"7 and "Santen"8, with the latter now the leading decision. Explicitly critical of the logic in Neurim, the Santen decision effectively returns the interpretation of Article 3(d) to that of Yissum. In short:
The first marketing authorisation is the earliest marketing authorisation for the active ingredient(s), irrespective of the disease indication or any other characteristics such as formulation, dose regime or patient group.
The UK courts have confirmed post-Brexit that they will continue to follow Santen (and not revert to Neurim)9. However, the Swiss courts and legislature have yet to apply Santen, and so for practical purposes Neurim is still in effect in Switzerland. Please ask your usual J A Kemp contact for more information on the opportunities that may exist to pursue additional "Neurim SPCs" in Switzerland based on later marketing authorisations of additional indications.
How many SPCs may be granted for a given product or patent?
Although Article 3(c) of the SPC Regulation suggests that only one SPC can be granted for a given product, it has long been the case that if two basic patents are owned by different patentees, each patentee can secure an SPC. Under such circumstances, both SPCs can be based on the same marketing authorisation.
If two patents which cover a given product are held by a single patentee, only one SPC is available. The patentee must choose which patent to use to support the SPC. Considerations which may apply when determining which patent to choose will include the relative vulnerability of the patents to any validity challenge, the suitability of the patent claims for SPC purposes (see section on Article 3(a) above), and the duration of the SPC available from each patent.
In general, it is also possible to have multiple SPCs granted for multiple different products on the basis of the same basic patent, provided that each product is protected by the basic patent10. The CJEU has on several occasions also considered whether a granted SPC for a single active ingredient A precludes under Article 3(c) the grant of a later SPC for a combination containing that active ingredient, A+B. The most recent decision in Teva/Merck18 has clarified that the only test for Article 3(c) is whether or not the precise product (i.e. the active ingredient or specific combination of active ingredients) for which SPC protection is sought has already been the subject of an SPC. No other assessment of the content of the basic patent is required. Of course, satisfying Article 3(c) via this commendably simple test does not guarantee that the more complex test for compliance with Article 3(a) will be met (see the relevant section above).
Is it possible for a third party to challenge the grant of the SPC?
Even where there is no formal mechanism to do so, most national patent offices will consider observations filed by a third party against an application for an SPC. Such observations may draw the Examiner's attention to deficiencies in the application with respect to compliance with the substantive requirements of the SPC Regulation. Such observations may slow (or even prevent) grant of the SPC application. After grant, the validity of an SPC may be challenged in the national courts on the same grounds.
The validity of the basic patent underlying an SPC may of course also be challenged separately via all normal routes (EPO opposition, national revocation action etc.). Should the patent be revoked, any SPC or SPC application based upon it is automatically invalidated.
Additional considerations in the UK post-Brexit
Post-Brexit, the basic requirements for grant of SPCs in the UK are essentially the same as in the EU, because the EU's SPC Regulation was transposed into UK law as part of the Brexit Withdrawal Agreement. In fact, the EU SPC Regulation continues to apply directly to SPCs which were pending or granted prior to the end of the Brexit implementation period on 31 December 2020. Similarly, CJEU case law prior to this date is directly applicable to SPCs in the UK, but subsequent case law is not directly applied and deviation has become possible.
Furthermore, although examination of SPC applications and grant in the UK will be based on the first marketing authorisation in the UK, the term of the granted SPC will be calculated based on the first authorisation in EU/EEA or UK. The manufacturing waiver in the UK will apply for export outside of the UK and EU, with stockpiling for sale in UK or EU permitted within the final 6 months.
However, the legal framework for the authorisation of medicines in the UK has undergone a series of changes post-Brexit. Since these changes influence the issue of marketing authorisations for the UK, they are directly relevant to SPCs. There are three distinct periods. The first and last / current periods are relatively simple:
First period: EU law applied in the UK until the end of the Brexit implementation period on 31 December 2020. For practical purposes, this means that the EU Regulation operated directly for pending and granted cases filed up to 31 December 2020, including the applicability of paediatric extensions and the SPC manufacturing waiver. SPC applications could be based on marketing authorisations issued by the European Medicines Agency (EMA), which were automatically converted into suitable authorisations covering the whole UK.
Current period: From 1 January 2025 the UK regulatory body (the MHRA) became the only body competent to authorise medicines in the UK (i.e. Great Britain and Northern Ireland). This means that SPC applications filed after this date in the UK can only be based on a marketing authorisation from the MHRA.
Unfortunately the period between the first period and the current period, i.e. from 1 January 2021 to 31 December 2024, is more complicated. During this middle period, the UK operated a dual regulatory regime, under which Northern Ireland (NI) remained subject to EU regulatory laws, whereas the rest of the UK (Great Britain; GB) was subject only to national law. For most drugs authorised in this period, there were therefore two relevant marketing authorisations:
- UK(NI) – compliant with EU law, granted by the EMA and effective only for NI
- UK(GB) – compliant with GB law, granted by the MHRA and effective only for GB
This did not mean separate SPCs. Rather, a single SPC application was filed (and a single SPC granted) based on whichever authorisation(s) the applicant held prior to the relevant deadline for filing the application. The SPC application (or granted SPC) would then have a geographical scope limited to the available authorisations. Up to entry into force of the SPC, it was permitted to file any additional marketing authorisation (GB or NI) to make a complete set and cover the whole UK. As of 1 January 2025 any UK(GB) authorisation automatically expanded to cover NI as well, and so any SPC based on such authorisation would automatically extend to cover the whole UK.
Annex
Regulation (EC) No 469/2009 of the European Parliament and of the Council of 6 May 2009 (selected provisions)
Article 1
Definitions
For the purposes of this Regulation, the following definitions shall apply:
- 'medicinal product' means any substance or combination of substances presented for treating or preventing disease in human beings or animals and any substance or combination of substances which may be administered to human beings or animals with a view to making a medical diagnosis or to restoring, correcting or modifying physiological functions in humans or in animals;
- 'product' means the active ingredient or combination of active ingredients of a medicinal product; [...]
Article 2
Scope
Any product protected by a patent in the territory of a Member State and subject, prior to being placed on the market as a medicinal product, to an administrative authorisation procedure as laid down in Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community code relating to medicinal products for human use or Directive 2001/82/EC of the European
Parliament and of the Council of 6 November 2001 on the Community code relating to veterinary medicinal products may, under the terms and conditions provided for in this Regulation, be the subject of a certificate.
Article 3
Conditions for Obtaining a Certificate
A certificate shall be granted if, in the Member State in which the application referred to in Article 7 is submitted and at the date of that application:
- the product is protected by a basic patent in force;
- a valid authorisation to place the product on the market as a medicinal product has been granted in accordance with Directive 2001/83/EC or Directive 2001/82/EC, as appropriate;
- the product has not already been the subject of a certificate;
- the authorisation referred to in point (b) is the first authorization to place the product on the market as a medicinal product.
Article 4
Subject matter of protection
Within the limits of the protection conferred by the basic patent, the protection conferred by a certificate shall extend only to the product covered by the authorisation to place the corresponding medicinal product on the market and for any use of the product as a medicinal product that has been authorised before the expiry of the certificate.
Footnotes
1 SPCs are also available for active substances in plant protection products, but these are not the focus of this briefing.
2 The purpose is thus similar to Patent Term Extensions (PTE) available in e.g. USA and Japan, but unlike those systems the SPC is not an extension of a patent, it is a separate right.
3 The CJEU held in C-431/04 MIT (relating to a bioerodible matrix) and C-210/13 GlaxoSmithKline (relating to an adjuvant in a vaccine) that the respective substances did not qualify as "active ingredients". In C-631/13 Forsgren, the CJEU held that to qualify as an "active ingredient", it must be established that a substance "produces a pharmacological, immunological or metabolic action of its own which is covered by the therapeutic indications of the marketing authorisation".
4 The UK officially left the EU at 11pm UK time on 31 January 2020, at which point it entered an implementation (or transition) period. During the implementation period, EU law relevant to SPCs continued to apply directly in the UK. The implementation period ended at 11pm UK time on 31 December 2020, at which point the corresponding provisions transposed into UK national law came into effect.
5 Swiss marketing authorisations also automatically take effect in Liechtenstein, which unlike Switzerland is an EEA member. Thus this must be taken into account when determining the first authorisation in the EU/EEA. See comments on additional term provided by an SPC below.
6 C-322/10 Medeva, C-422/10 Georgetown and C442/11 Novartis v Actavis.
7 C-392/97 Farmitalia.
8 Pharmaq v Intervet 15-170539ASD-BORG/01.
9 Similar status to the CJEU for matters referred by the national courts of the EEA states: Norway, Iceland, Liechtenstein.
10 The waiver does not apply to SPCs which were already in force on 1 July 2019. For SPCs which were applied for before 1 July 2019 but which came into force after that date, the waiver is applicable but only from 2 July 2022.
11 The CJEU confirmed in C-471/14 Seattle Genetics that the relevant date of an EU Marketing Authorisation is the date on which notification of the authorisation is made to the holder (typically a few days later than the Commission Decision granting the authorisation). This may result in extra days of SPC term, although some national patent offices have yet to correct the term of SPCs granted prior to the CJEU decision. The further decision C-492/16 Incyte confirms that it is possible for a proprietor of an SPC already granted with an expiry date that is not in accordance with the principles set out in Seattle Genetics to request correction of the expiry date before the relevant national patent office.
12 C-181/95 Biogen v SKB explicitly endorsed this view.
13 It was confirmed in C-567/16 Merck that an "end of procedure" notice (indicates an MA is imminent) is not sufficient to support an SPC, and that the absence of a granted MA cannot be remedied later, since this is an irregularity in the underlying "product" rather than a defect in the "SPC application", and thus is not correctable.
14 C-322/10 Medeva.
15 C-493/12 Eli Lilly.
16 C-121/17 Teva.
17 C650/17 Royalty Pharma.
18 C-119/22 & C-149/22 (joined cases) Teva/Merck.
J A Kemp LLP acts for clients in the USA, Europe and globally, advising on UK and European patent practice and representing them before the European Patent Office, UKIPO and Unified Patent Court. We have in-depth expertise in a wide range of technologies, including Biotech and Life Sciences, Pharmaceuticals, Software and IT, Chemistry, Electronics and Engineering and many others. See our website to find out more.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.


